
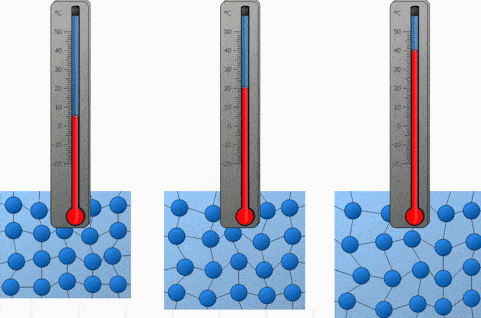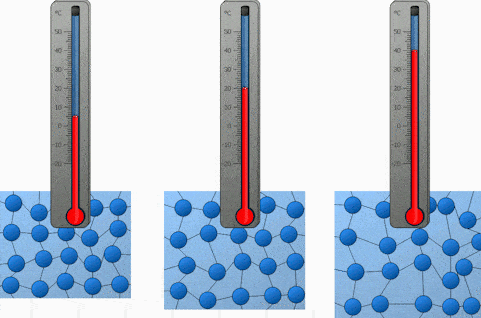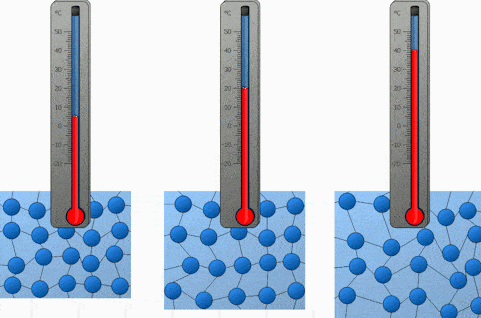
This means that the electric field produced by a charged biological macromolecule in salt solution is different from the electric field it would produce in vacuum. However, there is such an enormous volume of salt solution that can accommodate these extra negative ions that they can just spread out basically evenly and have very little effect. Not shown in the picture is that correspondingly, somewhere else there are extra negative ions. The result is a high concentration of positively charged ions immediately adjacent to the DNA, and a decreasing concentration as distance from the DNA increases. This weak field in turn attracts in some more positively charged ions, and the field of these ions further reduces the electric field. Thus outside the cloud, there is a weak electric field produced by the combination of the negatively charged DNA and this cloud of positively charged ions. But that even distribution has the highest entropy and thus is the most entropically favored.Ĭonsequently, what actually happens is a balance of these two effects as shown in the figure.** A “cloud” of positively charged ions clusters near the DNA, but there aren’t enough to completely neutralize the DNA. However, bringing these ions closer to the DNA molecule modifies the even distribution of positive and negative ions throughout the solution.

Without considering temperature and entropy, we’d expect the same scenario as with the charged object embedded in metal: positively charged ions would attach to the DNA molecule all along its length until it was electrically neutral. However, with the DNA molecule, positively charged ions are attracted to it and negatively charged ions repelled. Without the DNA molecule, the salt solution will have equal concentrations of positive and negative ions everywhere, so that there are no electric fields (on the average).
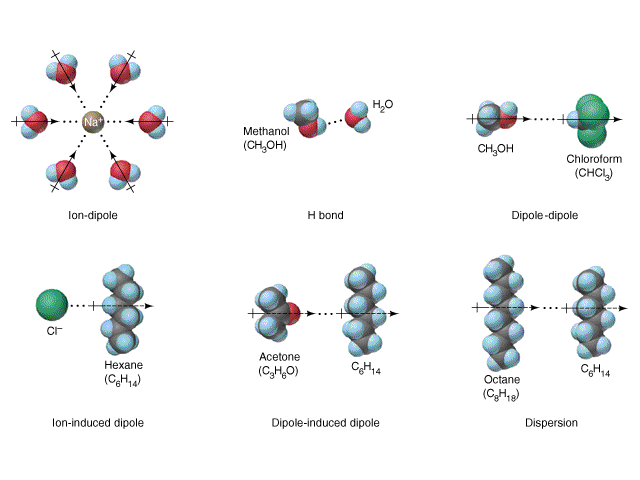
Let’s consider a negatively charged DNA molecule in an ionic salt solution. The effect that tends to spread the ions out, is entropy.Ĭonsequently, when a charged object, such as a protein (most proteins are negatively charged under physiological conditions) or a DNA molecule, is immersed in an ionic solution, a process happens called screening or shielding. Even if two charged particles are attracted together electrically, thermal motion can separate them if the thermal energy is greater than the electric potential energy associated with keeping them together. We have to take into account both the electrical forces on the dissolved ions, and the thermal energy that the water molecules and ions have that keeps them in steady motion. In this case, an additional effect matters. What matters are the many dissolved ions (K +, Na +, Cl -, OH -, H +.
#WATER IN MOTION CREATES NEGATIVE IONS FREE#
In an ionic fluid, like salt water, the relevant charges are not free electrons. Our treatment of charges and a conductor is a toy model. In metals, for reasons that require some pretty sophisticated physics to explain, we can ignore the thermal motion of electrons in conductors, and explain the electrical behavior of electrons in metals entirely in terms of electrical forces.* Once there is no field inside the material, and hence no force, there is no further rearrangement. These carriers therefore would move until the external field and the field due to the rearranged mobile charge carriers add to zero everywhere inside the material.
(“In the bulk” means everywhere in the material except right at the surface.) This happens because if there were a nonzero electric field in the bulk of the material, then that field would exert a force on the mobile charge carriers. (See Electric fields in matter.) We reasoned there that charge will rearrange on the conductor so that the total electric field in the bulk of the object is zero: the field due to the rearranged charge perfectly cancels the external field. We also considered what happens when a very good conductor, such as a metal, placed in an external electric field (“external” just means its sources are outside the conductor). We found in this case that the electric field of a spherical charged object, such as an ion or a globular protein, depends on distance away according to $1/r^2$, and the corresponding electric potential depends on distance according to $1/r$: We began with considering the electric forces and field produced by charged objects in vacuum. So far we’ve considered two kinds of environments in which electrical interactions occur.


 0 kommentar(er)
0 kommentar(er)
